Translating Causation To Treatment - Reviewing The Microbiome's Role In Drug Development
Translating Causation To Treatment - Reviewing The MicroBiome's Role In Drug Development
The gut microbiome continues to be at the fore front of medical research and both the industrial and academic communities expect that insights gained from microorganisms will soon become a critical component of health management across a gamut of critical indications. However, the biggest hurdle in translating promising gut microbiome research into effective therapeutic candidates is demonstrating the causative role of human microbiome in disease pathology. Although there are a growing number of microbiome-based candidates showing promising results in both pre-clinical and clinical settings, the approaches that are likely to have well-validated mechanisms of action are limited. The purpose of this theme will be to review the current thinking in approaching the human microbiome mechanistically and understanding both the academic and industrial perspectives on this important topic.
8.30
Microbiome Industry Leaders Panel Discussion
Bernat Olle
CEO
Vedanta Biosciences
Colleen Cutcliffe
CEO
Whole Biome
Eric Shaff
EVP & CEO
Seres Therapeutics
Ken Blount
Chief Scientific Officer
Rebiotix
Alison Lawton
CEO
Kaleido Biosciences
9.30
Healthy Infants Harbour Intestinal Bacteria That Protect Against Food Allergy
Cathryn Nagler
Bunning Food Allergy
Professor
University of Chicago
10.00
Microbiome Tools & Trends for the Pharmaceutical Industry
Julia Cope
Director of Scientific
Operations
Diversigen
10.30
Shotgun Sequencing & Strain-Level Analysis: Study Design for Pre-Clinical & Clinical Development
Nur Hasan
Chief Scientific Officer
CosmosID
11.00
Morning Refreshments and Speed Networking
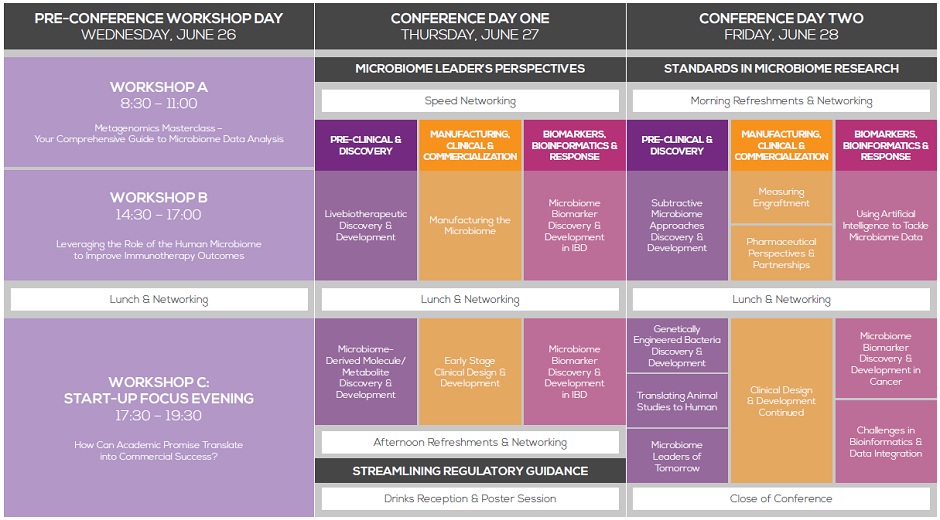
STREAM A: DISCOVERY PLATFORMS &
PRE-CLINICAL DEVELOPMENT OF
MICROBIOME-BASED THERAPEUTICS
STREAM B: MANUFACTURING, CLINICAL
DEVELOPMENT & COMMERCIALIZATION OF
MICROBIOME-BASED THERAPEUTICS
STREAM C: MICRONIOME BIOMARKERS, BIONFORMATICS & THERAPEUTIC RESPONSE
Introduction & Purpose: Prior to Clinical Validation, translational microbiome researchers look to leverage cutting edge pre-clinical models to identifymicrobiomederived targets that can be pursued for therapeutic development. The purpose of this stream will be to explore the discovery and pre-clinical assessment of microbiome-based therapeutics across the critical product modalities and disease phenotypes.
Introduction & Purpose: Although there has been significant progress both commercially and academically, there is still much we do not know about the microbiome and its role in disease causation. The purpose of this stream will be to review the critical product development challenges when bringing a microbiome-based therapeutic candidate to market. biomarker discovery and applications within the
Introduction & Purpose: As molecular biomarkers continue to become an important component to diagnosing disease and predicting response to treatment, the human microbiome has become a rich source for biomarker discovery across a broad range of disease phenotypes. The purpose of this stream will be to review efforts in biomarker discovery and applications within the drug development and diagnostic industry.
LIVEBIOTHERAPEUTIC DISCOVERY & DEVELOPMENT
MANUFACTURING THE MICROBIOME/
FORMULATION AND DELIVERY
MICROBIOME BIOMARKER DISCOVERY
& DEVELOPMENT IN IBD
12.00 - A Preclinical Assessment of a Rationally Designed LBP
Esi Lamouse-Smith
Director Early Development
Translational Medicine in Immunology
Janssen Pharmaceuticals
12.30 - Translating Probiotic Research into LBP Drug Programs
Nigel Titford
Chief Executive Officer
Biogaia Pharma
12.00 - Panel Discussion - Manufacturing and Scaling LBPs
Adrien Nivoliez
Chief Executive Officer
biose industrie
Bharat Dixit
VP Bioprocess & Analytical Development
Finch Therapeutics Group
Chris Reyes
CSO
Bloom Science
12.00 - Microbiome-based Biomarkers for Inflammatory Bowel Diseases: Opportunities to Accelerate Drug Development and Improve Clinical Outcomes
Gerard Honig
Translational Research Manager
Crohn’s & Colitis Foundation
12.30 - Utilizing Microbiome-Derived Biomarker to Assess PK/PD and Support the Development of Rationally Designed LBPs
Matthew Henn
EVP Microbiome R&D
Seres Therapeutics
13.00 - Developing Microbial Therapies to Prevent and Treat Human Disease and Allergies
Greg Mckenzie
Chief Scientific Officer
ConsortiaTX
12.30 - CMC Development for a Full Spectrum Microbiota Product
Bharat Dixit
VP Bioprocess & Analytical Development
Finch Therapeutics Group
13.00 - Microbiome-derived Biomarker Initiatives at Janssen Pharmaceuticals to Evaluate the Success of VE202
Ling-Yang Hao
Associate Director
Janssen Pharmaceuticals
13.30 - Delivery, Survival, Engraftment & Activity of LBPs in the GI Tract
Massimo Marzorati
Chief Executive Officer
ProDigest
13.00 - Development of Oral LBPs
– a CDMOs Perspective
Mike Frodsham
Pharmaceutical Development Manager
Quay Pharma
13.30 - Scale Up & GMP Manufacture of LBPs
Tina Chritensen-Ram
Quality Manager
LuinaBio
13.30 - Commercial Speaking Position Reserved for Solution/Service Provider
Please Contact adam.harasgummer
@hansonwade.com for further details
13.40
Lunch and Networking
14.40 - Improving Cancer Treatment Through Oncobax-Based Therapeutics
Romain Daillere
Co-Founder & Head of Preclinical Research
EverImmune
EARLY STAGE CLINICAL DESIGN & DEVELOPMENT
14.40 - Presentation Title TBC, Please Check Back with Us Soon
Trevor Lawley
Chief Scientific Officer
Microbiotica
SMALL MOLECULE/METABOLITE DISCOVERY
& DEVELOPMENT
14.40 - Clinical Development and Scale-Up Considerations for a Microbiome-based Therapeutic for the Treatment of Metabolic Disease
Gregory Lambert
Chief Executive Officer
TargEDys
15.10 - Commercial Speaking Position Reserved for Solution/Service Provider - Please Contact adam.haras-gummer@hansonwade.com for Further Details
15.10 - Commercial Speaking Position Reserved for Solution/Service Provider - Please Contact adam.haras gummer@hansonwade.com for Further Details
15.10 - Clinical Validation for Microbiome Modulators
Herwig Bachmann
Group Leader Fermentation
NIZO
15.40 - A Novel Machine Learning Approach to Microbiome Based Biomarker Discovery
Assaf Oron
Chief Business Officer
BiomX
15.40 - Microbiota-Derived Metabolites to Treat Food Allergy and Prevent Food Allergen Sensitivity
John Colson
Director of Operations
ClostraBio
15.40 - Reverse Translation for Therapeutic Development in the Human Microbiome
Ulrich Thienel
Chief Medical Officer
Finch Therapeutics Group
16.10 - The Growing Role of the Gut Microbiome in Drug Metabolism
Andrew Goodman
Associate Professor
Yale University
16.10 - A Chemistry-Driven Approach to Leveraging the Potential of the Microbiome Organ to Treat Disease and Improve Human Health
Katharine Knobil MD
Chief Medical Officer and Head of R&D
Kaleido Biosciences
16.10 - Developing Microbiome-based Therapeutics for the Prevention and Treatment of
Inflammatory Diseases
Nikole Kimes
Founder & Chief Scientific Officer
Siolta Therapeutics
16.40 - Commercial Speaking Position Reserved for Solution/Service Provider
- Please Contact adam.haras-gummer@hansonwade.com for Further Details
16.40 - From Sample to Data - Considerations for Designing Microbiome Studies
Malcolm Kendall
Chief Executive Officer
Microbiome Insights
16.40 - Overcoming Hurdles in the Development of Live Biotherapeutic Products
Stacy Burns-Guydish,
Founder & VP, Biotherapeutics Development & Manufacturing
List Laboratories
16.50
Afternoon Refreshments and Networking
STREAMLINING REGULATORY APPROVAL OF MICROBIOME-BASED THERAPEUTICS
CHATHAM HOUSE RULES C-LEVEL ROUNDTABLE (INVITE ONLY)(INVITE
As microbiome therapies constitute a novel paradigm in modern medicine, a robust regulatory framework thataddresses key safety and biocontainment issues should be evaluated for safe implementation of these treatments. Despite the rise in notoriety across the pharmaceutical industry, only a few regulatory agencies have provided clarity over the regulatory hurdles when developing a microbiome-based therapeutic. The purpose of this theme will be to outline how leading regulatory bodies currently review microbiome candidates whilst highlighting successful IND applications for emerging and well established microbiome companies.
17.15 – 18.45
This theme will be an invite only session that will look to unite the leaders of the translational microbiome space to discuss the long term challenges and opportunities that still lie within this ever-growing field.
17.15
Regulatory Considerations for LBPs & Bacteriophage Therapy
Representative from FDA Invited, More Details to Be Announced after Government Shutdown
17.45
Application of Multivalent Vaccine Manufacturing Testing Schemes in cGMP Characterization of Microbiome Defined Drug Products
Jeffrey Heiser
Director Microbiology
Boston Analytical
18.15
Panel Discussion – Building Clear Regulatory Guidelines for Microbiome-based Therapeutics
Representative from FDA Invited, More Details to Be Announced after Government Shutdown
Jim Weston
SVP Regulatory Affairs
Seres Therapeutics
Susan Stewart
SVP, Regulatory Affairs & Quality
Kaleido Biosciences
Kris Piper
VP Regulatory Affairs
Finch Therapeutics Group
Edward Burd
Head of Regulatory Affairs
Rebiotix
18.45
Evening Drinks Reception and Poster Session Hosted by The Microbiome Movement
20.00
Close of Conference Day One