About Event
The cell, gene and RNA industries are going through a period of intense growth and rapid advancement, with 1,500+ cell, gene and RNA therapies in clinical trials as of February 2023. With DNA vaccines making steady progress too, the demand for DNA as a critical material has soared.
While traditional DNA production methods are well understood, they have not evolved over the last decade to keep pace with this demand. Costs, manufacturing time, and loss of DNA over the production process result in a clear trade-off between quantity and quality of the final DNA obtained. The lack of regulatory guidance has further exacerbated this problem as biopharma moves to scale up DNA production platforms.
The DNA Process Development & Manufacturing Summit bridges the gap between the supply and demand for DNA, covering everything from step-wise process development to analytical assays and regulatory compliance.
With dedicated sessions on novel synthetic DNA technology, regulatory guidance, and 7+ hours of built-in networking time so you can continue the conversation into the breaks, this is the ultimate forum to de-risk your production platforms and advance your DNA-dependent therapeutics to the clinic faster.
Our 2024 Audience Joined Us To:
- Obtain regulatory advice and the latest guidelines to prepare for IND and clinical approvals with industry experts experienced in compliance from Bristol Myers Squibb, and US Pharmacopeia
- Learn about innovative new technologies and platforms to produce plasmid and cell-free DNA faster and for cheaper with insights from Genentech, and Mediphage Bioceuticals
- Optimize plasmid design, lysis protocols, and chromatographic techniques to maximize plasmid recovery and start with a high yield of superior quality DNA with Merck & Co and Alexion Pharmaceuticals
- Tailor your DNA production to mRNA-based approaches by creating a robust production and analytical platform to produce your drug faster with BioNTech and Sanofi
- Make an informed decision on how to balance internal and external production capacity to lower cost and time for DNA production Janssen R&D, and Nykode
Therapeutics
Who Joined Us:
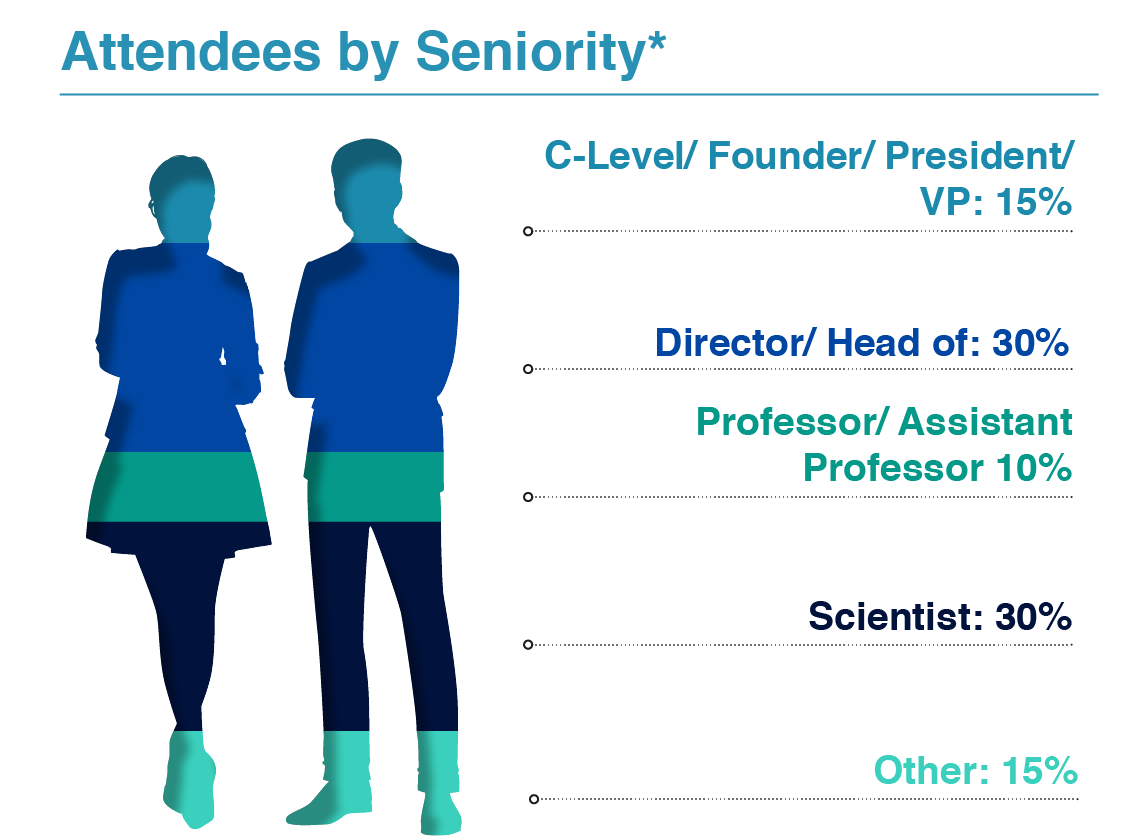
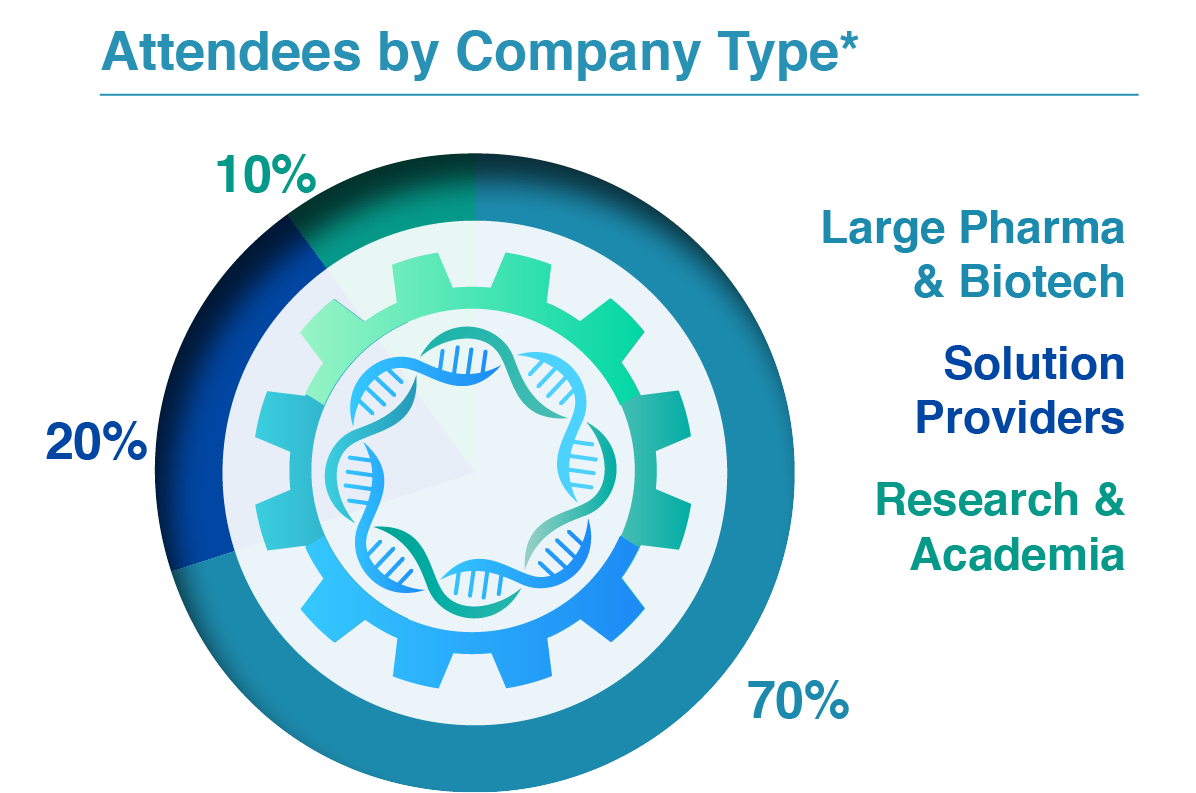
Are you looking to meet your fellow Directors, Heads, and Principal Scientists invested in DNA process development, analytical development, regulatory affairs, and CMC?
Join us as this niche community of large pharma and innovative biotech gathers over 3 days to assess the emerging technologies in the field, take back actionable insights to your team, and meet the rising demand for DNA production.
2024 Companies in the Room:

What Your Peers Have to Say:

"The big value I see in this meeting is to learn and get the word out about new DNA technologies and their benefits and limitations"
Steven Hersch
Senior Scientist - Molecular Genetics, Mediphage Bioceuticals

